NT-proANP ELISA/氨基端前心钠肽ELISA检测试剂盒
NT-proANP ELISA/氨基端前心钠肽ELISA检测试剂盒
加入购物车
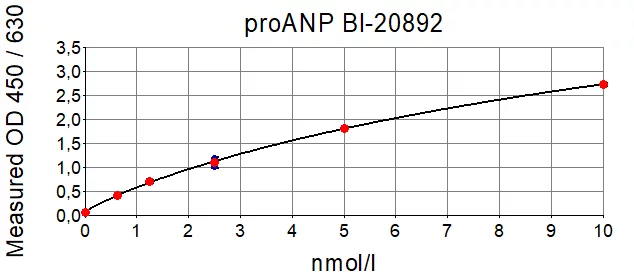
产品概述
N-terminal proatrial natriuretic peptide (NT-proANP 1-98) is the biologically inactive fragment (98 amino acid) of the ANP prohormone.
ANP is translated from its gene as a 151-amino acid precursor, pre-proANP. After removal of the 25-amino acid signal peptide, the tissue form of the hormone, a 126-amino acid proANP (γ-ANP) is generated. γ-ANP is proteolytically cleaved to the biologically active ANP (α-ANP) and to the 98 amino acid NT-proANP peptide (also known as proANP 1-98). The major molecular forms of circulating human ANP is thus the 28 amino-acid peptide (α-ANP) that contains a ring structure with a disulfide bridge and the 98-amino acid NT-proANP peptide, which is easier to detect in the circulation due to its longer half-life. Both peptides α-ANP and NT-proANP circulate in equimolar amounts.
ANP is secreted from the heart in response to atrial stretching or through stimulation by angiotensin II and endothelin. The most important stimulus for the release of the hormone into circulation is stretching of myocyte fibres. On release, the prohormone is split into equimolar amounts of the highly biologically active proANP (99-126), also known as -ANP or ANP, and the N-terminal part proANP (1-98) (also known as NT-proANP) (Nakagawa et al., 2019; Volpe et al., 2015).
NT-proANP is more stable and has a longer half-life (60-120 min) in circulation than ANP which is rapidly cleared from the circulation with a half-life of 3-4 minutes (Yandle et al., 1986). The natriuretic peptides have a common characteristic biochemical structure that consists of a ring of 17 amino acids and a disulfide bridge between 2 cysteine molecules (Clerico et al., 2011; Volpe et al., 2015; Yandle et al., 1986).
The ANP prohormone undergoes several cleavages to generate the biologically active form of the hormone. N-terminal (NT) prohormone fragments of natriuretic peptides are typically more stable, have longer half-lives, and circulate at higher concentrations compared to C-terminal biologically active hormone ends (Yandle et al., 1986). It has therefore been suggested that measuring NT prohormone fragments of ANP provide more accurate concentrations in samples (Clerico et al., 2011).
The expression and secretion of ANP increase significantly in pathological states accompanied by stretching the heart chambers, volume overload, and ischemic injury, such as heart failure and myocardial infarction.

.jpg)
.jpg)